Accuracy of the Brighton Pediatric Early Warning Score in detecting clinical deterioration events among pediatric patients: retrospective cohort study
SPMC J Health Care Serv. 2025;11(1):8 ARK: https://n2t.net/ark:/76951/jhcs4aw6a9
1Department of Pediatrics, Southern Philippines Medical Center, JP Laurel Ave, Davao City, Philippines
Correspondence Giselle Godin, Giselle.Godin@gmail.com
Received 13 March 2024
Accepted 17 June 2025
Cite as Godin G, Cansino-Valeroso MA, Dadia DM. Accuracy of the Brighton Pediatric Early Warning Score in detecting clinical deterioration events among pediatric patients: retrospective cohort study. SPMC J Health Care Serv. 2025;11(1):8. https://n2t.net/ark:/76951/jhcs4aw6a9
Introduction
PEWS have been adopted by many hospitals worldwide to predict clinical deterioration and guide decisions regarding escalation of care. 3 4 6 These systems are clinical assessment tools that use vital signs along with patient signs and symptoms to effectively detect clinical deterioration.5 7 8 In the ED setting, PEWS complement existing triage systems by helping to prioritize patients and potentially predict the need for admission to a pediatric intensive care unit (PICU) or other specialized units.9 10
The PEWS developed by Monaghan et al. in 2005 at the Royal Alexandra Hospital for Sick Children, Brighton, is one such scoring system designed for pediatric acute care settings. It evaluates three components--behavior, cardiovascular status, and respiratory status—each scored from 0 to 3, with a total of 9 points. A high score indicates poor clinical status,8 11 which correlates with adverse outcomes among pediatric patients.12 13
The effectiveness of PEWS in enhancing patient outcomes depends on various factors, including the specific setting, study population, specific interventions, and outcome measures used.6 We conducted this study to determine the optimal cut-off point of the Brighton PEWS for predicting CDE among pediatric patients seen in the SPMC ED, and to determine the sensitivity, specificity, and positive and negative likelihood ratios of this threshold.
Methodology
To determine the minimum sample size for this study, we assumed a specificity of 91% for PEWS ≥3 in predicting ICU admission.14 Using a sample size calculation for diagnostic accuracy studies--with a 5% allowable difference in specificity, a significance level of 5%, and a power of 80%--the minimum required sample size was determined to be 313.
Results
| Table 1 Demographic and clinical profile of pediatric patients with versus without clinical deterioration events (CDE) | |||||
| Characteristics | n | Without CDE | n | With CDE | p-value |
|---|---|---|---|---|---|
| Median age (IQR), years | 289 | 5.00 (1.00 to 12.00) | 56 | 1.00 (0.08 to 10.50) | <0.001*† |
| Sex, frequency (%) | 289 | 56 | 0.254 | ||
| Male | 162 (56.06) | 36 (64.29) | |||
| Female | 127 (43.94) | 20 (35.71); | |||
| Z-score, frequency (%) | 289; | 56 | 0.110‡ | ||
| -3 | 43 (14.88) | 14 (25.00) | |||
| -2 | 39 (13.49) | 6 (10.71) | |||
| -1 | 96 (33.22) | 22 (39.29) | |||
| 0 | 27 (9.34) | 1 (1.79) | |||
| 1 | 66 (22.84) | 8 (14.29) | |||
| 2 | 8 (2.77) | 3 (5.36) | |||
| 3 | 10 (3.46) | 2 (3.57) | |||
| Comorbidities, frequency (%) | 289 | 56 | <0.001* | ||
| None | 158 (54.67) | 18 (32.14) | |||
| Cardiovascular disease | 7 (2.42) | 3 (5.36) | |||
| Metabolic disease | 5 (1.73) | 6 (10.71) | |||
| Neurologic disease | 14 (4.84) | 4 (7.14) | |||
| Renal disease | 25 (8.65) | 15 (26.79) | |||
| Respiratory disease | 42 (14.53) | 7 (12.50) | |||
| Congenital disease | 8 (2.77) | 0 (0.00) | |||
| COVID-19 infections | 21 (7.27) | 3 (5.36) | |||
| Others | 9 (3.11) | 0 (0.00) | |||
| Median mean arterial pressure (IQR) | 200 | 70.00 (70.00 to 80.00) | 24 | 70.00 (70.00 to 87.00) | 0.879† |
| Median heart rate (IQR), BPM | 288 | 117.00 (101.00 to 133.50) | 56 | 135.00 (111.00 to 158.00) | <0.001*† |
| Median temperature (IQR), ℃ | 289 | 36.80 (36.60 to 37.20) | 56 | 36.60 (36.25 to 37.10) | 0.065† |
| Median respiratory rate (IQR),bpm | 289 | 24.00 (22.00 to 30.00) | 56 | 32.50 (28.00 to 46.00) | <0.001*† |
| Median oxygen saturation (IQR),% | 289 | 98.00 (97.00 to 99.00) | 56 | 93.00 (89.00 to 97.00) | <0.001*† |
| Median pGCS (IQR) | 289 | 15.00 (15.00 to 15.00) | 56 | 8.00 (6.00 to 13.00) | <0.001*† |
| Diagnosis, frequency (%) | 289 | 56 | <0.001* | ||
| Pediatric community acquired pneumonia | 41 (14.19) | 19 (33.93) | |||
| Dengue fever | 53 (18.34) | 0 (0.00) | |||
| Acute gastroenteritis | 45 (15.57) | 7 (12.50) | |||
| Neonatal sepsis | 31 (10.73) | 11 (19.64) | |||
| Febrile convulsions | 14 (4.84) | 1 (1.79) | |||
| Meningitis | 3 (1.04) | 10 (17.86) | |||
| Bronchial asthma in acute exacerbation | 6 (2.08) | 0 (0.00) | |||
| Oncologic illness | 40 (13.84) | 6 (10.71) | |||
| COVID-19 | 20 (6.92) | 0 (0.00) | |||
| Others | 36 (12.46) | 2 (3.57) | |||
| Median Brighton PEWS (IQR) | 289 | 2.00 (1.00 to 3.00) | 56 | 6.00 (4.00 to 7.00) | <0.001*† |
|
IQR=interquartile range; BPM=beats per minute; bpm=breaths per minute; pGCS=pediatric Glasgow Coma Scale; PEWS=pediatric early warning score
*Significant at p<0.05 †Rank sum test ‡Fisher's exact |
|||||
 |
|
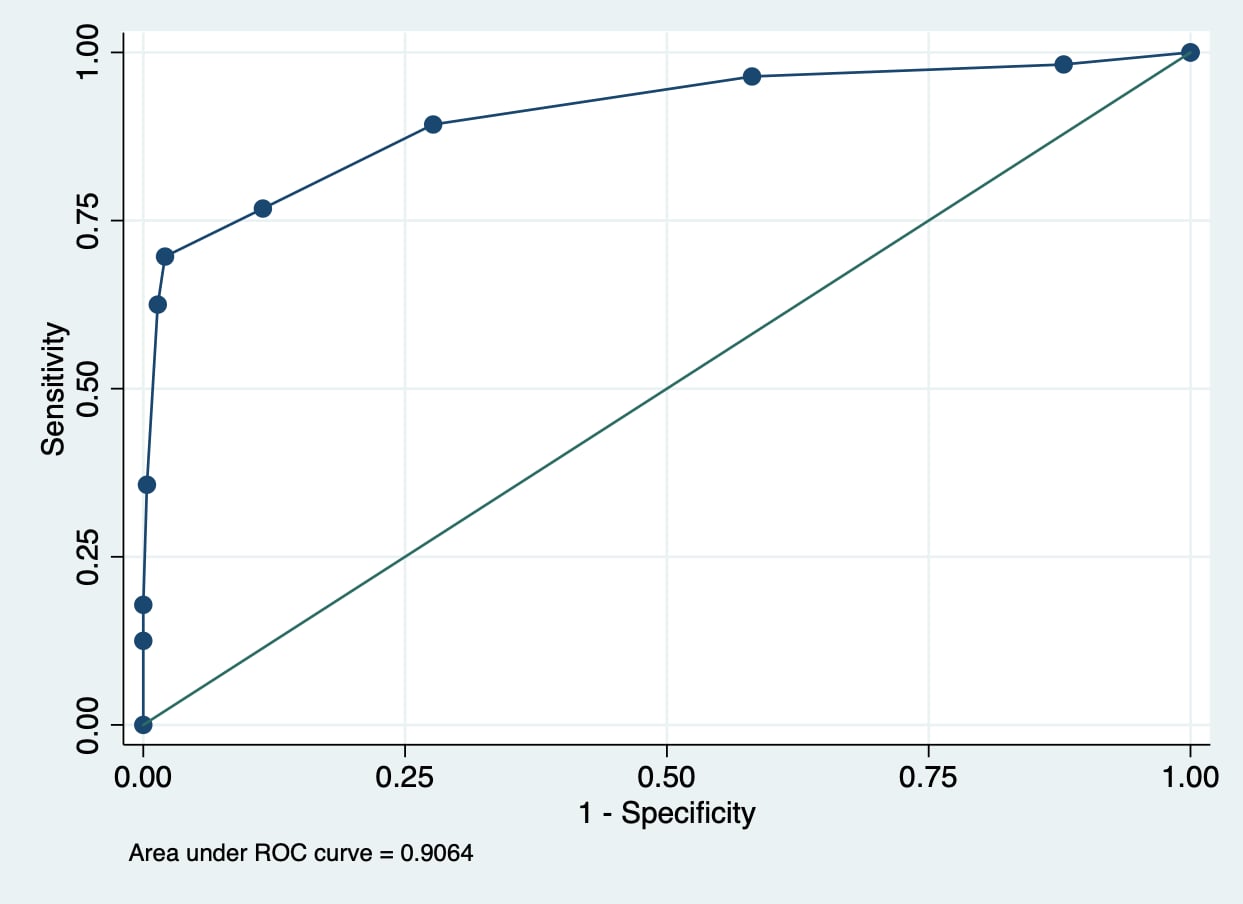
Figure 1 Receiver operator characteristic (ROC) curve of the Brighton Pediatric Early Warning Score (PEWS) in detecting clinical deterioration events (CDE). |
| Table 2 Optimal cut-off point and accuracy of the Brighton PEWS in detecting CDE | ||||||
| Characteristics | Values | |||||
|---|---|---|---|---|---|---|
| Optimal cut-off point | 4.00* | |||||
| Sensitivity | 76.79% | |||||
| Specificity | 88.58% | |||||
| Positive predictive value | 56.60% | |||||
| Negative predictive value | 95.20% | |||||
| Positive likelihood ratio | 6.72 | |||||
| Negative likelihood ratio | 0.26 | |||||
| *Youden index | ||||||
Discussion
In this study, using a cut-off of ≥4.00, we found a balanced trade-off between sensitivity and specificity in predicting CDE. Similar studies showed that among pediatric patients, a Brighton PEWS cut-off of 4.00 was optimal for detecting the outcome ‘major intervention required,’ a cut-off of 2.00 is optimal in detecting ICU admission, and a cut-off of 5.00 demonstrated good accuracy in detecting mortality.12 15
While PEWS serves as a valuable tool for early recognition of clinical deterioration in children,23 it is essential that its cut-off scores are rigorously validated for the population in which they are applied. Improper implementation may lead to misclassification--either underestimating severity, which delays care, or overestimating it, which can lead to unnecessary interventions and resource strain. Careful validation and context-appropriate use of PEWS are critical to maximizing its clinical benefit.24
Conclusion
Contributors
GG, MACV, and DMD had substantial contributions to the study design, and to the acquisition, analysis and interpretation of data. GG wrote the original draft and subsequent revisions. All authors reviewed, edited, and approved the final version of the manuscript. All authors agreed to be accountable for all aspects of the work.
Ethics approval
This study was reviewed and approved by the Davao Center for Health Development Joint Research Ethics Committee JREC-202384.
Reporting guideline used
Article source
Submitted
Peer review
External
Funding
Supported by personal funds of the authors
Competing interests
None declared
Access and license
This is an Open Access article licensed under the Creative Commons Attribution-NonCommercial 4.0 International License, which allows others to share and adapt the work, provided that derivative works bear appropriate citation to this original work and are not used for commercial purposes. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc/4.0/.
References
1 Lambert V, Matthews A, MacDonell R, Fitzsimons J. Paediatric early warning systems for detecting and responding to clinical deterioration in children: a systematic review. BMJ Open. 2017 Mar 13;7(3):e014497.
2 Rørbech JT, Jensen CS, Dreyer P, Herholdt-Lomholdt SM. Beyond objective measurements: Danish nurses' identification of hospitalized pediatric patients at risk of clinical deterioration - A qualitative study. J Pediatr Nurs. 2022 Sep-Oct;66:e67-e73.
3 Chapman SM, Wray J, Oulton K, Pagel C, Ray S, Peters MJ. 'The Score Matters': wide variations in predictive performance of 18 paediatric track and trigger systems. Arch Dis Child. 2017 Jun;102(6):487-495. doi: 10.1136/archdischild-2016-311088. Epub 2017 Mar 14.
4 Mayampurath A, Jani P, Dai Y, Gibbons R, Edelson D, Churpek MM. A Vital Sign-Based Model to Predict Clinical Deterioration in Hospitalized Children. Pediatr Crit Care Med. 2020 Sep;21(9):820-826.
5 Teheux L, Verlaat CW, Lemson J, Draaisma JMT, Fuijkschot J. Risk stratification to improve Pediatric Early Warning Systems: it is all about the context. Eur J Pediatr. 2019 Oct;178(10):1589-1596.
6 Chong SL, Goh MSL, Ong GY, Acworth J, Sultana R, Yao SHW, et al. Do paediatric early warning systems reduce mortality and critical deterioration events among children? A systematic review and meta-analysis. Resusc Plus. 2022 Jun 29;11:100262.
7 Murray JS, Williams LA, Pignataro S, Volpe D. An Integrative Review of Pediatric Early Warning System Scores. Pediatr Nurs. 2015 Jul-Aug;41(4):165-74.
8 Monaghan A. Detecting and managing deterioration in children. Paediatr Nurs. 2005 Feb;17(1):32-5.
9 Cheng Y, Zhang X, Zhang J, Lu G. The application of pediatric early warning score (PEWS) in emergency observation room. J Pediatr Nurs. 2022 Sep-Oct;66:1-5.
10 Parwaiz A, Agrawal N, Gupta A, Simalti A, Kedarnath M. Utility of pediatric early warning score at emergency room in predicting the level of care required for next 48 h: A single-center, prospective, observational study. Journal of Pediatric Critical Care. 2024;11(1):15-18.
11 Elencwajg M, Grisolía NA, Meregalli C, Montecuco MA, Montiel MV, Rodríguez GM, et al. Usefulness of an early warning score as an early predictor of clinical deterioration in hospitalized children. Arch Argent Pediatr. 2020 Dec;118(6):399-404.
12 Gold DL, Mihalov LK, Cohen DM. Evaluating the Pediatric Early Warning Score (PEWS) system for admitted patients in the pediatric emergency department. Acad Emerg Med. 2014 Nov;21(11):1249-56.
13 Bambo. Validation of the Pediatric Early Weaning Score (PEWS) in Timely Detection of Clinical Deterioration among Children Admitted to Non-ICU Areas of a Tertiary Hospital in the Philippines. UPMREB Registration No. 2012-229. UP-PGH. 2012.
14 Chaiyakulsil C, Pandee U. Validation of pediatric early warning score in pediatric emergency department. Pediatr Int. 2015 Aug;57(4):694-8. doi: 10.1111/ped.12595. Epub 2015 Apr 28.
15 Malde S, Jain P, Revathi N, Seth B, Setia MS. Evaluation of Pediatric Early Warning Score (PEWS) in Unintentional Childhood Injuries Admitted to the Critical Care Unit. Cureus. 2024 Jul 24;16(7):e65312.
16 Breslin K, Marx J, Hoffman H, McBeth R, Pavuluri P. Pediatric early warning score at time of emergency department disposition is associated with level of care. Pediatr Emerg Care. 2014 Feb;30(2):97-103.
17 Tucker KM, Brewer TL, Baker RB, Demeritt B, Vossmeyer MT. Prospective evaluation of a pediatric inpatient early warning scoring system. J Spec Pediatr Nurs. 2009 Apr;14(2):79-85.
18 Seiger N, Maconochie I, Oostenbrink R, Moll HA. Validity of different pediatric early warning scores in the emergency department. Pediatrics. 2013 Oct;132(4):e841-50. doi: 10.1542/peds.2012-3594. Epub 2013 Sep 9. PMID: 24019413.
19 Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009 Feb;37(2):666-88.
20 Carcillo JA, Fields AI; American College of Critical Care Medicine Task Force Committee Members. Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med. 2002 Jun;30(6):1365-78.
21 Thompson M, Coad N, Harnden A, Mayon-White R, Perera R, Mant D. How well do vital signs identify children with serious infections in paediatric emergency care? Arch Dis Child. 2009 Nov;94(11):888-93.
22 Mann KD, Good NM, Fatehi F, Khanna S, Campbell V, Conway R, et al. Predicting Patient Deterioration: A Review of Tools in the Digital Hospital Setting. J Med Internet Res. 2021 Sep 30;23(9):e28209.
23 Mills D, Schmid A, Najajreh M, Al Nasser A, Awwad Y, Qattush K, et al. Implementation of a pediatric early warning score tool in a pediatric oncology Ward in Palestine. BMC Health Serv Res. 2021 Oct 26;21(1):1159.
24 Lillitos PJ, Maconochie IK. Paediatric early warning systems (PEWS and Trigger systems) for the hospitalised child: time to focus on the evidence. Arch Dis Child. 2017 Jun;102(6):479-480.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution-NonCommercial 4.0 International License that allows others to share the work for non-commercial purposes with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional, non-commercial contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors grant the journal permission to rewrite, edit, modify, store and/or publish the submission in any medium or format a version or abstract forming part thereof, all associated supplemental materials, and subsequent errata, if necessary, in a publicly available publication or database.
- Authors warrant that the submission is original with the authors and does not infringe or transfer any copyright or violate any other right of any third parties.
